The objective of the activity is devoted to one of the key points to corroborate the experimental results. With the available sensors, a mass balance can be performed within the amount of deuterium injected and extracted. Thus, the deuterium inventory in the circuit and the performance of the extractor unit is obtained indirectly. However, more accurate results can be obtained by an online measurement of deuterium concentration within the liquid metal. There are no commercial sensors capable of being used in this harsh environment. Thus, a dedicated research is being made to fabricate ad-hoc sensors. Two different technologies are being assessed: one based on the same principle of permeation against vacuum and the other based on the electrochemical detection.
- O2.1- H-Sensor based on permeation In the LML, a sensor based on PAV technology has been tested. It is made of a Pd/Ag capillary designed to maximize the permeation surface. The validation of the sensor in gas-phase has already been performed and an updated prototype has been manufactured to improve the operation under a PbLi flow. Experiments in static and dynamic PbLi are the focus of this objective. As in the extraction process, hydrogen permeates through the Pd/Ag membrane and the vacuum system carries it to a quadrupole mass spectrometer for its detection.
- O2.2- H-Sensor based on electrochemical detection The electrochemical online-monitoring of hydrogen species in aqueous systems is already a well-established feature worldwide, with typical response times of milliseconds. Electrochemical sensors (ECHS) are commonly composed by two electrodes -one of them is a reference electrode-, an electrolyte and a semipermeable membrane. Two different designs are under evaluation depending on the selected membrane: a solid permeable or a semi porous polycrystalline material. The membrane must be resistant to corrosion generated by PbLi and by the selected electrolyte, (permeable to the target species -hydrogen isotopes- and electric insulating), such as the niobium (other materials are being explored). The reference electrode must have a stable, fixed and well-known electrode potential (lithium - lithium hydride). The supportive electrolyte, as the medium, must provide the adequate chemical and physical properties to guarantee the ionic mobility (calcium chloride + calcium hydride). After the selection of the membrane materials, the fabrication of the sensor will be performed. The capsule of the material will be assembled with the electrolytes and the electrodes assuring the tightness. The detection is performed by measuring the voltage due to potential difference between the reference and counter electrodes.
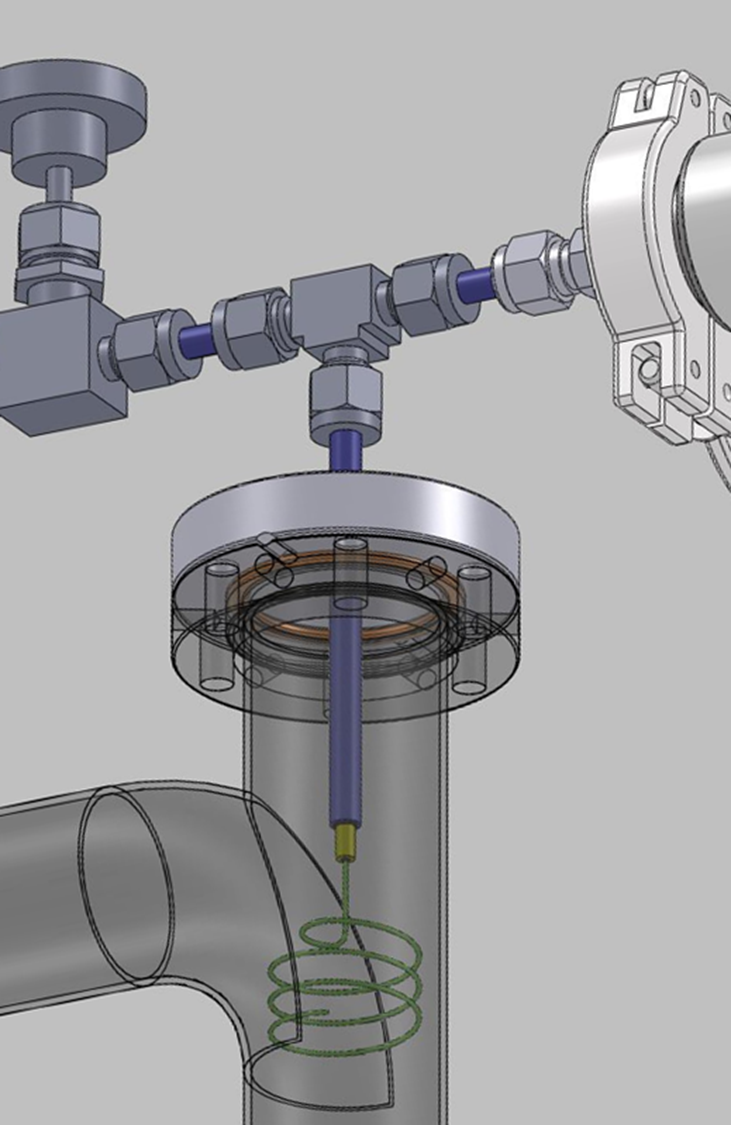
Figure. PAV-based sensor scheme
